February 14, 2004
Eheheh
Ok sorry about that last post. Let's just say I got about 4 hours of sleep before my neighbor's alarm clock went off for at least an hour. It really irked me. I couldn't sleep. And I couldn't shut it off.
Thanks to Borst for linking me to this comic.
In any case, I have an interesting story about Friday the 13th. I had chem lab (polymer lab) on Friday the 13th; I didn't even realize the date until the lab had ended.
The lab started off as normal; we got a lab report back, we turned one in, we took a quiz, and we got started on our lab.
But things were not to be good. First off, for whatever reason, the argon gas tank was broken. We had no argon gas, and since we had to cannulate and put stuff under positive pressure a few times, we needed the gas.
So we called over the lab manager, who said that she needed to "boys" to "open" the tank. So I got shafted with holding the tank while another guy got a huge wrench to try to open the tank (we tried to open the tank with our hands but our hands kept chafing; the thing was stuck hard).
Now, the tank is located in a really ackward place and holding the tank while someone uses a wrench to open it was really straining on my back; this is how I threw my back out yesterday. After a while, they realized that "hey maybe the tank is broken and won't open!"
After a half hour of waiting, a new tank was brought in. And then we got to start cannulating. As I mentioned earlier, we had to put stuff under positive pressure (e.g. just put stuff in capped containers to higher pressures than the atmosphere). Of course, one of the cap ends wasn't tightened enough, so air started escaping when we cannulated, which started making this high-pitched noise, almost like an alarm.
We were so embarassed.
Anyways, this lab then required the injections of two chemicals that are water sensitive. This inherently means the chemical is air-sensitive (since there is water in the air) ... which means there is a really specific method you have to use in order to extract the chemical from the vial and then inject it.
One group managed to inject the first chemical alright. Then the second group that got the chemicals screwed up.
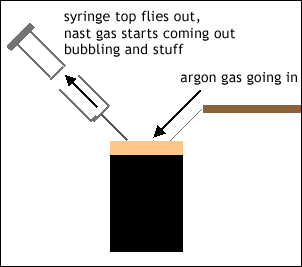
The method involves you putting the capped original chemical vial under positive pressure with argon, sticking a syringe in, then extracting the chemical, pulling the syringe out, then pulling the argon gas out. Unfortunately when they stuck the syringe in, they left the part of the syringe that goes up and down in the syringe; it should of been left out so it doesn't fly out.
Because the top part FLEW out ... into a bucket of ... ice. Remember this is the water-sensitive chemical. This basically meant that this syringe was wasted and had to be washed with acetone and dried in an oven forever (since that was the only syringe we had).
And to make matters worse, when the top part of the syringe flew off, the group kinda just stared at the set-up. Out of syringe was now the water/air sensitive chemical ... bubbling out. It looked like all those nasty chemicals in movies; it was yellow and bubbling out, and then it would just turn into smoke ... it was pretty scary. (Look at the picture attached to this entry below).
But this isn't the end! No sirree!
The first group, if you remember, managed to get their first chemical in. But they couldn't get their second chemical in. The syringe for that kept bending; it could penetrate the rubber top on the second chemical jar! Ha ha! Then the lab manager came over and was like "just stab it in there and extract the chemical; don't worry about the air.
And then my TA was like, "Uhh, the chemical is air sensitive." The lab manager's just like "Oh, it's ok. Just pump it a few times in the jar when argon's flowing in.
That doesn't make much sense it seems. In any case, I'll cut this story short cause I'm getting hungry. We ended up giving up on all 4 groups trying to make the polymer; we decided to just let the first group try. But as it turned out, the step that the lab manager "helped" on leaded to no product being yielded.
Fun stuff.
In any case, I have an interesting story about Friday the 13th. I had chem lab (polymer lab) on Friday the 13th; I didn't even realize the date until the lab had ended.
The lab started off as normal; we got a lab report back, we turned one in, we took a quiz, and we got started on our lab.
But things were not to be good. First off, for whatever reason, the argon gas tank was broken. We had no argon gas, and since we had to cannulate and put stuff under positive pressure a few times, we needed the gas.
So we called over the lab manager, who said that she needed to "boys" to "open" the tank. So I got shafted with holding the tank while another guy got a huge wrench to try to open the tank (we tried to open the tank with our hands but our hands kept chafing; the thing was stuck hard).
Now, the tank is located in a really ackward place and holding the tank while someone uses a wrench to open it was really straining on my back; this is how I threw my back out yesterday. After a while, they realized that "hey maybe the tank is broken and won't open!"
After a half hour of waiting, a new tank was brought in. And then we got to start cannulating. As I mentioned earlier, we had to put stuff under positive pressure (e.g. just put stuff in capped containers to higher pressures than the atmosphere). Of course, one of the cap ends wasn't tightened enough, so air started escaping when we cannulated, which started making this high-pitched noise, almost like an alarm.
We were so embarassed.
Anyways, this lab then required the injections of two chemicals that are water sensitive. This inherently means the chemical is air-sensitive (since there is water in the air) ... which means there is a really specific method you have to use in order to extract the chemical from the vial and then inject it.
One group managed to inject the first chemical alright. Then the second group that got the chemicals screwed up.
The method involves you putting the capped original chemical vial under positive pressure with argon, sticking a syringe in, then extracting the chemical, pulling the syringe out, then pulling the argon gas out. Unfortunately when they stuck the syringe in, they left the part of the syringe that goes up and down in the syringe; it should of been left out so it doesn't fly out.
Because the top part FLEW out ... into a bucket of ... ice. Remember this is the water-sensitive chemical. This basically meant that this syringe was wasted and had to be washed with acetone and dried in an oven forever (since that was the only syringe we had).
And to make matters worse, when the top part of the syringe flew off, the group kinda just stared at the set-up. Out of syringe was now the water/air sensitive chemical ... bubbling out. It looked like all those nasty chemicals in movies; it was yellow and bubbling out, and then it would just turn into smoke ... it was pretty scary. (Look at the picture attached to this entry below).
But this isn't the end! No sirree!
The first group, if you remember, managed to get their first chemical in. But they couldn't get their second chemical in. The syringe for that kept bending; it could penetrate the rubber top on the second chemical jar! Ha ha! Then the lab manager came over and was like "just stab it in there and extract the chemical; don't worry about the air.
And then my TA was like, "Uhh, the chemical is air sensitive." The lab manager's just like "Oh, it's ok. Just pump it a few times in the jar when argon's flowing in.
That doesn't make much sense it seems. In any case, I'll cut this story short cause I'm getting hungry. We ended up giving up on all 4 groups trying to make the polymer; we decided to just let the first group try. But as it turned out, the step that the lab manager "helped" on leaded to no product being yielded.
Fun stuff.
Thanks to Borst for linking me to this comic.
In any case, I have an interesting story about Friday the 13th. I had chem lab (polymer lab) on Friday the 13th; I didn't even realize the date until the lab had ended.
The lab started off as normal; we got a lab report back, we turned one in, we took a quiz, and we got started on our lab.
But things were not to be good. First off, for whatever reason, the argon gas tank was broken. We had no argon gas, and since we had to cannulate and put stuff under positive pressure a few times, we needed the gas.
So we called over the lab manager, who said that she needed to "boys" to "open" the tank. So I got shafted with holding the tank while another guy got a huge wrench to try to open the tank (we tried to open the tank with our hands but our hands kept chafing; the thing was stuck hard).
Now, the tank is located in a really ackward place and holding the tank while someone uses a wrench to open it was really straining on my back; this is how I threw my back out yesterday. After a while, they realized that "hey maybe the tank is broken and won't open!"
After a half hour of waiting, a new tank was brought in. And then we got to start cannulating. As I mentioned earlier, we had to put stuff under positive pressure (e.g. just put stuff in capped containers to higher pressures than the atmosphere). Of course, one of the cap ends wasn't tightened enough, so air started escaping when we cannulated, which started making this high-pitched noise, almost like an alarm.
We were so embarassed.
Anyways, this lab then required the injections of two chemicals that are water sensitive. This inherently means the chemical is air-sensitive (since there is water in the air) ... which means there is a really specific method you have to use in order to extract the chemical from the vial and then inject it.
One group managed to inject the first chemical alright. Then the second group that got the chemicals screwed up.
The method involves you putting the capped original chemical vial under positive pressure with argon, sticking a syringe in, then extracting the chemical, pulling the syringe out, then pulling the argon gas out. Unfortunately when they stuck the syringe in, they left the part of the syringe that goes up and down in the syringe; it should of been left out so it doesn't fly out.
Because the top part FLEW out ... into a bucket of ... ice. Remember this is the water-sensitive chemical. This basically meant that this syringe was wasted and had to be washed with acetone and dried in an oven forever (since that was the only syringe we had).
And to make matters worse, when the top part of the syringe flew off, the group kinda just stared at the set-up. Out of syringe was now the water/air sensitive chemical ... bubbling out. It looked like all those nasty chemicals in movies; it was yellow and bubbling out, and then it would just turn into smoke ... it was pretty scary. (Look at the picture attached to this entry below).
But this isn't the end! No sirree!
The first group, if you remember, managed to get their first chemical in. But they couldn't get their second chemical in. The syringe for that kept bending; it could penetrate the rubber top on the second chemical jar! Ha ha! Then the lab manager came over and was like "just stab it in there and extract the chemical; don't worry about the air.
And then my TA was like, "Uhh, the chemical is air sensitive." The lab manager's just like "Oh, it's ok. Just pump it a few times in the jar when argon's flowing in.
That doesn't make much sense it seems. In any case, I'll cut this story short cause I'm getting hungry. We ended up giving up on all 4 groups trying to make the polymer; we decided to just let the first group try. But as it turned out, the step that the lab manager "helped" on leaded to no product being yielded.
Fun stuff.
In any case, I have an interesting story about Friday the 13th. I had chem lab (polymer lab) on Friday the 13th; I didn't even realize the date until the lab had ended.
The lab started off as normal; we got a lab report back, we turned one in, we took a quiz, and we got started on our lab.
But things were not to be good. First off, for whatever reason, the argon gas tank was broken. We had no argon gas, and since we had to cannulate and put stuff under positive pressure a few times, we needed the gas.
So we called over the lab manager, who said that she needed to "boys" to "open" the tank. So I got shafted with holding the tank while another guy got a huge wrench to try to open the tank (we tried to open the tank with our hands but our hands kept chafing; the thing was stuck hard).
Now, the tank is located in a really ackward place and holding the tank while someone uses a wrench to open it was really straining on my back; this is how I threw my back out yesterday. After a while, they realized that "hey maybe the tank is broken and won't open!"
After a half hour of waiting, a new tank was brought in. And then we got to start cannulating. As I mentioned earlier, we had to put stuff under positive pressure (e.g. just put stuff in capped containers to higher pressures than the atmosphere). Of course, one of the cap ends wasn't tightened enough, so air started escaping when we cannulated, which started making this high-pitched noise, almost like an alarm.
We were so embarassed.
Anyways, this lab then required the injections of two chemicals that are water sensitive. This inherently means the chemical is air-sensitive (since there is water in the air) ... which means there is a really specific method you have to use in order to extract the chemical from the vial and then inject it.
One group managed to inject the first chemical alright. Then the second group that got the chemicals screwed up.
The method involves you putting the capped original chemical vial under positive pressure with argon, sticking a syringe in, then extracting the chemical, pulling the syringe out, then pulling the argon gas out. Unfortunately when they stuck the syringe in, they left the part of the syringe that goes up and down in the syringe; it should of been left out so it doesn't fly out.
Because the top part FLEW out ... into a bucket of ... ice. Remember this is the water-sensitive chemical. This basically meant that this syringe was wasted and had to be washed with acetone and dried in an oven forever (since that was the only syringe we had).
And to make matters worse, when the top part of the syringe flew off, the group kinda just stared at the set-up. Out of syringe was now the water/air sensitive chemical ... bubbling out. It looked like all those nasty chemicals in movies; it was yellow and bubbling out, and then it would just turn into smoke ... it was pretty scary. (Look at the picture attached to this entry below).
But this isn't the end! No sirree!
The first group, if you remember, managed to get their first chemical in. But they couldn't get their second chemical in. The syringe for that kept bending; it could penetrate the rubber top on the second chemical jar! Ha ha! Then the lab manager came over and was like "just stab it in there and extract the chemical; don't worry about the air.
And then my TA was like, "Uhh, the chemical is air sensitive." The lab manager's just like "Oh, it's ok. Just pump it a few times in the jar when argon's flowing in.
That doesn't make much sense it seems. In any case, I'll cut this story short cause I'm getting hungry. We ended up giving up on all 4 groups trying to make the polymer; we decided to just let the first group try. But as it turned out, the step that the lab manager "helped" on leaded to no product being yielded.
Fun stuff.


Comment with Facebook
Want to comment with Tabulas?. Please login.
MacDaddyTatsu (guest)